A total of people have accessed this page since 3/9/99.
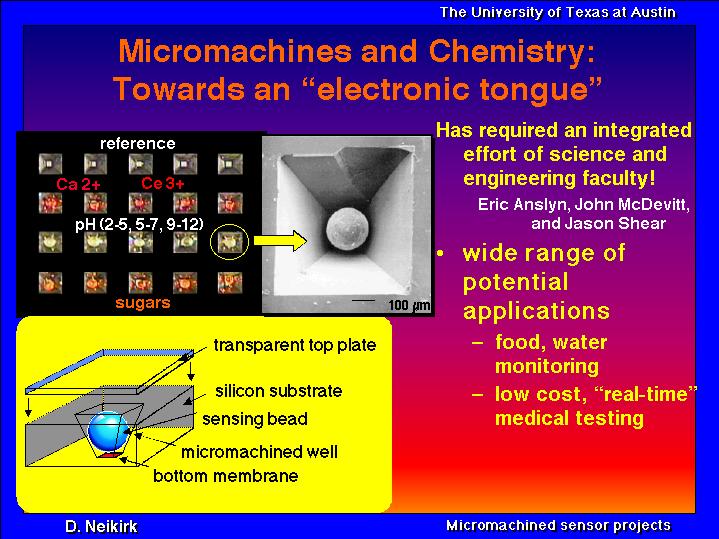
In collaboration with Eric Anslyn, John McDevitt, and Jason Shear, we are working on new chemical sensors. Shown below is a Quicktime movie of a polystyrene bead that has been "tagged" so that there is a very strong (and reversible) optical color change when the pH of the bead's environement changes. We are studying micromachined "micro-test tubes" and integrated optical interfaces to allow the compact packaging of arrays of such sensing beads.
Click on the image above to see a color movie of beads while solutions of changing pH are introduced (quick time, 625k).
Click on the image above to see a nicer color movie of an array, again responding to pH changes, but over a ten decade range in concentration (Real Video, streaming, 200k total). These movies were done by co-workers in John McDevitt's lab.
Click on the image above to see a Real Video stream of a bead placement operation (~300k total); placement and movie done by Youngsoo Sohn and Rob Friar.
to be submitted Chemistry of Materials (3/12/98)
Steve Savoy, John J. Lavigne, Jascinda Clevenger, J. Seung-Jin Yoo,
John T. McDevitt, Eric V. Anslyn, Jason B. Shear, Dean Neikirk
Contribution from Department of Chemistry and Biochemistry
Department of Electrical Engineering
The University of Texas at Austin
Austin, TX 78712
Abstract:
The development of a sensor array composed of individually immobilized polystyrene-polyethylene glycol composite microspheres has been demonstrated. The microspheres are selectively arranged in micromachined etch cavities localized on silicon wafers which have the rear face terminated with a Si3N4-SiO2-Si3N4 transparent membrane structure. The membrane acts as a window and enables studies of the optical properties of the sensing elements upon their exposure to analyte-carrying aqueous solutions. Sensing occurs via colorimetric or fluorometric changes to receptor molecules which are covalently bound to amine termination sites on the polymeric microspheres. The unique combination of carefully chosen reporter molecules with water permeable microspheres allows for the simultaneous detection and quantification of a variety of analytes. The fabrication of the sensor structure and the initial colorimetric and fluorescent responses using a charge-coupled device (CCD) detector for pH, Ca+2, Ce+3, and sugar are reported. In addition, response time for selected microsphere structures is described.
Also see:
For other information on some of our chemical sensor work, please see our paper:
J. Han, D. P. Neikirk, M. Clevenger, and J. T. McDevitt, "Fabrication and characterization of a Fabry-Perot based chemical sensor," SPIE Microelectronic Structures and MEMS for Optical Processing II, M. E. Motamedi and W. Bailey, editors, Proc. SPIE 2881, Austin, Texas, USA, 14-15 October, 1996, pp. 171-178.
Slide show on micromachined storage wells for an"electronic tongue" .
Return to research overview in the Microelectromagnetic Device Group